Background: Axicabtagene ciloleucel (axi-cel), an autologous chimeric antigen receptor (CAR) T-cell therapy, achieved an overall response rate (ORR) of 94% and a complete response (CR) rate of 79% in patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more prior lines of therapy in the ZUMA-5 trial ( Jacobson et al. Lancet Oncol. 2022). Since its FDA approval in March 2021, there has been little evidence on comparative effectiveness (CE) studies of axi-cel vs standard-of-care (SoC), except ones using ZUMA-5 and historical SCHOLAR-5 data (eg, Ghione et al. Blood. 2022). This study was aimed to address this evidence gap in CE of axi-cel vs SoC used to treat r/r FL in real-world settings.
Methods: The two data sources in this study were patients who received commercial axi-cel between March 2021 and May 2023 from the CIBMTR and patients who received historical SoC (eg, chemotherapy, anti-CD20 mAb + chemotherapy, immunomodulatory imide drugs) between July 2014 and December 2020 from SCHOLAR-5, which served as an external control of ZUMA-5 trial. The index date was defined as the initiation date of either the infusion for axi-cel patients or the last eligible systemic therapy for SoC patients. Patients were included if they were age ≥ 18, had documented r/r FL with histological grade 1, 2 or 3a, and received at least 2 prior lines of therapy at the index. Patients were excluded if they had transformed diffused large B-cell lymphoma, central nervous system involvement, prior receipt of CAR T or other non-CAR T cellular therapies or allogeneic stem cell transplant, or no post-index information on outcomes.
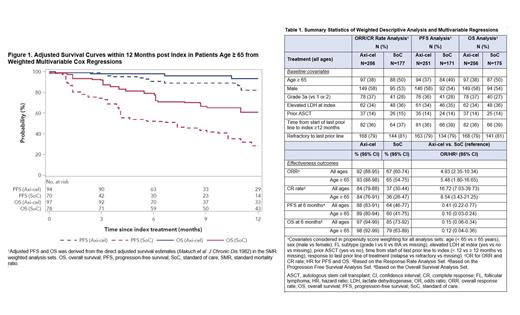
Four effectiveness outcomes were ORR, CR rate, progression-free survival (PFS), and overall survival (OS). Imbalance in observed prognostic risk factors between the two was adjusted via a propensity score analysis using the standard mortality ratio weighting (SMRW). The primary analyses included weighted univariable analysis and multivariable logistic (ORR and CR rate) or Cox (PFS and OS) regressions that were adjusted for the covariates after the SMRW. A sub-analysis of patients age ≥ 65 was conducted using the same methods as the primary analyses.
Results: The unweighted study cohort included 376 patients, in which 256 (68%) received axi-cel . Thepatients who received axi-cel were more likely to be younger (median age 61 for axi-cel vs 67 for SoC), have grade 3a FL (37% vs 12%), received 3 or more prior lines of therapy (83% vs 58%), or remained refractory to the last prior therapy (79% vs 73%) vs those who received SoC. The imbalance in the observed prognostic risk factors was mitigated via the SMRW.
The weighted univariable analysis, in which the sample size by treatment reflected the SMRW, is shown in Table 1. In patients of all ages, ORR was 92% in axi-cel vs 67% in SoC, and CR rate was 84% vs 37%. Because of varying follow-up lengths by treatment (median 7 months for axi-cel and 37 months for SoC), survival outcomes were reported at month 6, at which time PFS rate was 88% in axi-cel vs 64% in SoC and OS rate was 97% vs 85%. The corresponding proportions for patients age ≥ 65 were similar to those reported for patients of all ages.
In the weighted multivariable regressions, axi-cel was associated with statistically significantly better outcomes by all 4 effectiveness measures vs SoC (Table 1). Specifically, in patients of all ages, the odds ratios for axi-cel were 4.93 (95% CI, 2.35−10.34) for ORR and 16.72 (95% CI, 7.03−39.73) for CR rate, and the hazard ratios for axi-cel were 0.41 (95% CI, 0.22−0.77) for PFS and 0.15 (95% CI, 0.06−0.34) for OS. Similar to the patients of all ages in the primary analyses, patients age ≥ 65 in the sub-analysis also demonstrated improved outcomes associated with axi-cel (Figure 1).
Conclusions: This study showed that patients with r/r FL after two or more prior lines of therapy treated with axi-cel experienced better outcomes compared with patients treated with SoC, and that older patients also benefited significantly from axi-cel. These findings suggest that axi-cel addresses an important unmet medical need in patients with r/r FL. Future research should include longer follow-up data on outcomes of axi-cel and CE of axi-cel vs other contemporary SoC therapies.
Disclosures
Wang:Kite, a Gilead Company: Current Employment. Yan:Gilead Sciences: Current holder of stock options in a privately-held company; Kite, A Gilead Company: Current Employment. Herrera:AstraZeneca/MedImmune: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Regeneron: Consultancy; Takeda: Consultancy; Kite, a Gilead Company: Research Funding; Tubulis GmbH: Consultancy; Merck: Consultancy, Research Funding; Pfizer: Consultancy; Genentech/Roche: Consultancy, Research Funding; AbbVie: Consultancy; Genmab: Consultancy; Allogene Therapeutics: Consultancy; Caribou Biosciences: Consultancy; Karyopharm Therapeutics: Consultancy; Adicet Bio: Consultancy; BMS: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding; Seattle Genetics: Consultancy, Research Funding; Gilead Sciences: Research Funding; AstraZeneca: Research Funding. Frank:EcoR1: Consultancy; Kite, a Gilead Company: Research Funding; Adaptive Biotechnology: Consultancy; BRVLH: Consultancy; Cargo Therapeutics: Consultancy, Other: Travel Support; Gilead Sciences: Consultancy, Other: Travel Support; Allogene: Consultancy; Roche/Genentech: Current holder of stock options in a privately-held company. Popplewell:Pfizer: Honoraria; La Roche: Honoraria; Hoffmann: Honoraria; Seattle Genetics: Consultancy, Honoraria; Novartis: Consultancy. Ahmed:Bristol Myers Squibb: Consultancy; Kite, a Gilead company: Research Funding. Locke:National Cancer Institute: Other; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Society for Immunotherapy of Cancer: Other; BioPharma Communications CARE Education: Other: Institutional; Leukemia and Lymphoma Society: Other; Clinical Care Options Oncology: Other; EcoR1: Consultancy; Cellular Medicine Group: Consultancy; CERo Therapeutics: Other: (Institutional); Iovance: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional; GammaDelta Therapeutics: Consultancy; Aptitude Health: Other: Travel Support; Emerging Therapy Solutions: Consultancy, Other; Umoja: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sana: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Wugen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Legend Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imedex: Other; ASH: Other: Travel Support; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional ; Calibr: Consultancy; Caribou: Consultancy; Cowen: Consultancy; Daiichi Sankyo: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Bristol Myers Squibb/ Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Gerson Lehrman Group (GLG): Consultancy; A2 Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Ghione:Kyowa Hakko Kirin: Consultancy; Kite, A Gilead Company: Research Funding; AstraZeneca Pharmaceuticals: Consultancy; Secura Bio: Consultancy. Gribben:AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Speakers Bureau; Kite, A Gilead Company: Consultancy, Speakers Bureau; Janssen Pharmaceuticals, Inc: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Novartis: Consultancy. Best:Bristol Myers Squibb: Current holder of stock options in a privately-held company, Ended employment in the past 24 months, Other; Kite, a Gilead company: Current Employment. Fu:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Other; Amgen: Current holder of stock options in a privately-held company, Other; Cellares: Other: Intellectual property , Patents & Royalties. Beygi:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Other: Travel support. Ray:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Other: Current holder of stock in a privately-held company. Bian:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Ontada: Current Employment. Hu:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current holder of stock options in a privately-held company, Other. Sun:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current holder of stock options in a privately-held company, Other. Pasquini:Bristol Myers Squibb: Consultancy, Research Funding; Kite, a Gilead Company: Honoraria, Research Funding; Novartis: Research Funding; Janssen: Research Funding; Kite Brazil: Honoraria. Jacobson:ADC Therapeutics: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Abbvie: Consultancy; AstraZeneca: Consultancy; Abintus Bio: Consultancy; Caribou Bio: Consultancy; Instil Bio: Consultancy; ImmPACT Bio: Consultancy; Daiichi-Sankyo: Consultancy; Ipsen: Consultancy; Morphosys: Consultancy; Synthekine: Consultancy; Pfizer: Research Funding; Miltenyi Biotec: Consultancy; Novartis: Consultancy; Kite, a Gilead company: Consultancy, Research Funding.